Abstract
Introduction: Proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and treatments involving both a PI and an IMiD (PI+IMiD) are the principal therapies for treating relapsed/refractory multiple myeloma (RRMM). The immuno-oncology (I-O) agents elotuzumab (elo) and daratumumab (dara) may bring new and promising solutions to patients (pts). The adoption of these treatments may come with high healthcare resource utilization (HCRU) and costs. It is important to further understand HCRU by different treatment modalities in real-world practice settings. Here we evaluate HCRU and costs in pts receiving different treatment modalities for RRMM.
Methods: PREAMBLE is an ongoing, prospective, multinational, noninterventional observational study (NCT01838512). Pts ≥18 y of age with RRMM, ≥1 prior therapy, and who initiated treatment (index therapy) with a PI (including carfilzomib [carf], bortezomib [bort], and ixazomib), an IMiD (including lenalidomide [len], pomalidomide [pom], and thalidomide), an IMiD+PI combination, or I-O agents within 90 d before or 30 d after study consent were identified. Pt data were collected at each healthcare provider (HCP) visit, over 3 y or until the end of pt follow-up, including clinic/physician office visits, emergency room (ER) visits, hospital outpatient visits, hospitalizations, and home healthcare/other visits. Demographic and baseline characteristics were analyzed using descriptive statistics. The median duration of treatment (mDoT) was estimated using Kaplan-Meier (K-M) methods. HCRU was estimated using the mean number of incidents, per-1000-patients-per-month (1000 PPM) metric in a period up to 12 mo from start of index therapy (index date).
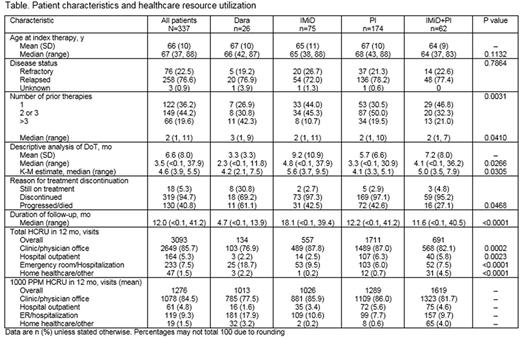
Results: 341 pts (median age 67 y; 59% male) were enrolled in the US. At the time of data cut-off (May 27, 2017), 187 (55%) had withdrawn from the study; 117 (63%) of the withdrawn pts had died. Median (range) follow-up was 11.8 mo (<0.1, 41.2). At study entry, 30 pts were receiving I-O agent-based index therapy (dara, n=26; elo, n=4). Pts were divided into 4 cohorts based on index therapy: dara (26/337, 8%), PI (n=174, 52%; carf 92/174; bort 80/174), IMiD (n=75, 22%; pom 31/75), and IMiD+PI (n=62, 18%; bort+len 35/62). The 4 treatment cohorts were similar in sex, race, disease status, International Staging System stage, and comorbidities, although pts on dara received significantly more prior therapies than those on other treatments (Table). Of the 26 pts on dara, 31% (n=8) were still on treatment at the time of data cut-off, and of those who discontinued index therapy (n=18, 69%), 61% (n=11) were due to disease progression, which was higher than for other treatments (p=0.0468). The K-M estimate of mDoT for pts on dara was 4.2 mo, similar to that for PI (4.1 mo) but shorter than for other treatments (p=0.0305). In the PI cohort, carf had a shorter mDoT than bort (3.4 vs 5.2 mo). Of 3093 HCP visits overall, the most common type was clinic/physician office visits (n=2649, 86%), followed by ER visits/hospitalizations (n=233, 8%), and hospital outpatients (n=164, 5%). Per 1000 pts treated, pts receiving PI+IMiD had the most HCRU visits in a 1-mo period, vs least for dara (mean 1000 PPM: 1619 vs 1013 visits). This was also true for clinic/physician office visits and hospital outpatients (Table). Notably, mean 1000 PPM ER visits/hospitalizations were higher for pts receiving dara than for any other treatment (n=181, 18%). Among pts receiving a PI, those on carf had higher mean 1000 PPM total visits (n=1001) than those on bort (n=710); this remained true for each of the healthcare resource categories investigated in this study.
Conclusions: Routine management of RRMM and treatment-related events drive HCRU, which may differ by treatment. This preliminary analysis suggests that pts on dara appear to have shorter mDoT and fewer outpatient visits, but more ER visits and hospitalizations, compared with pts on other treatments. Receiving carf appears to be associated with more HCRU visits overall, and for each of the healthcare resource categories compared with bort. The study is ongoing and further analysis will be necessary to better understand HCRU patterns observed with these new treatment modalities in clinical practice settings.
Study support: Bristol-Myers Squibb.
Kuter: Fujifilm: Consultancy; Dova: Consultancy, Membership on an entity's Board of Directors or advisory committees; ONO: Consultancy; 3SBIO: Consultancy; Protalex: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Rigel: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Merck: Consultancy; Syntimmune: Consultancy, Research Funding; Pfizer: Consultancy; Novartis: Consultancy; Zafgen: Consultancy; Genzyme: Consultancy; Shire: Consultancy, Research Funding; Amgen: Consultancy; Alexion: Consultancy, Research Funding; Argenx: Consultancy. Chen: Bristol-Myers Squibb: Employment. Popov: Parexel: Employment. Davis: Bristol-Myers Squibb: Employment. Vij: Takeda, Onyx: Research Funding; Celgene, Onyx, Takeda, Novartis, BMS, Sanofi, Janssen, Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal